Why do molecules make ions?
Why do molecules make ions?
So, let's try to come to know it in simple ways.
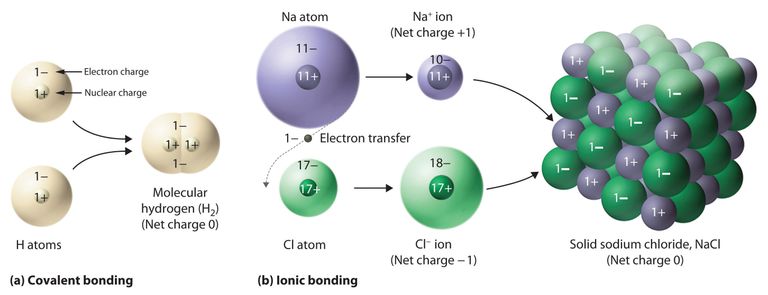
Now move to the original topic.
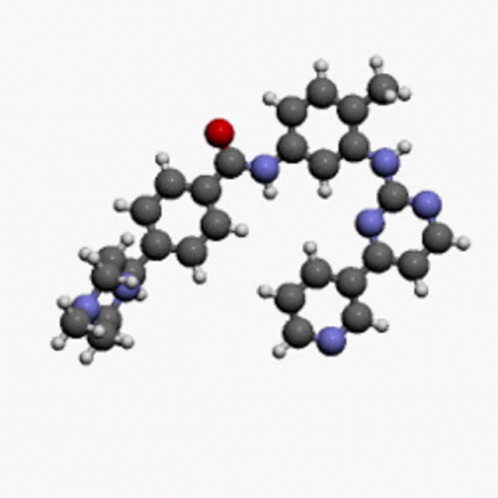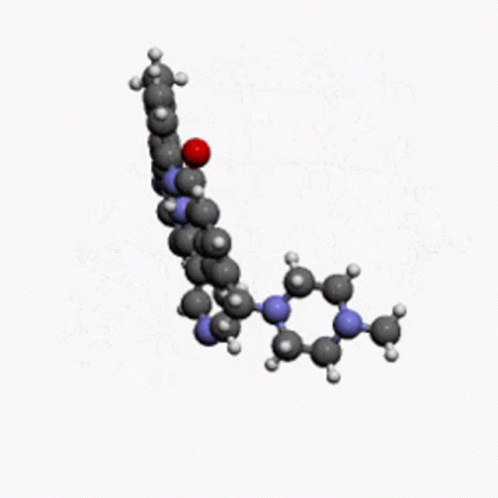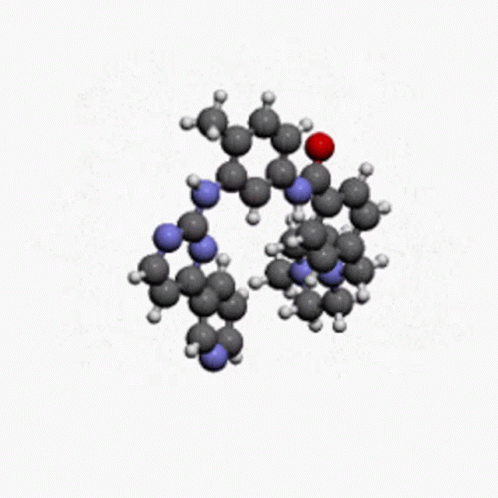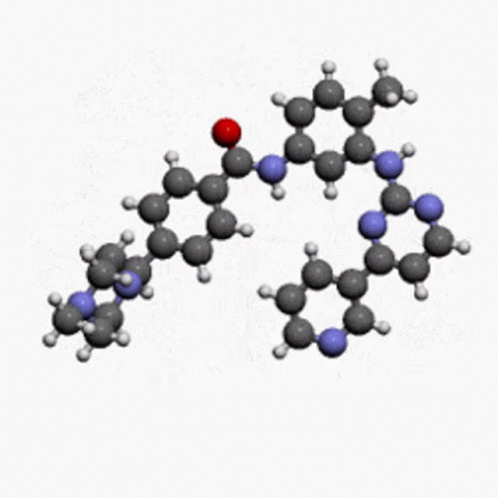
Molecules become from different atoms and sometimes they have a charge but our main question is that why they have a charge on them. I want to explain it with an example. Na+ have +ve charge and OH- have -ve charge. When they are combined then NaOH becomes a compound. Molecule of Na had charge and OH also had charge so when they are combined then NaOH become in the stable form.
So, we can say that molecules have the charge to combine in the form of an element.
Blog By.
@ghiasahmad
These are not many strong conclusions. So, help me to correct them in the case of a correction.