Let us learn the De Broglie hypothesis and matter wave.
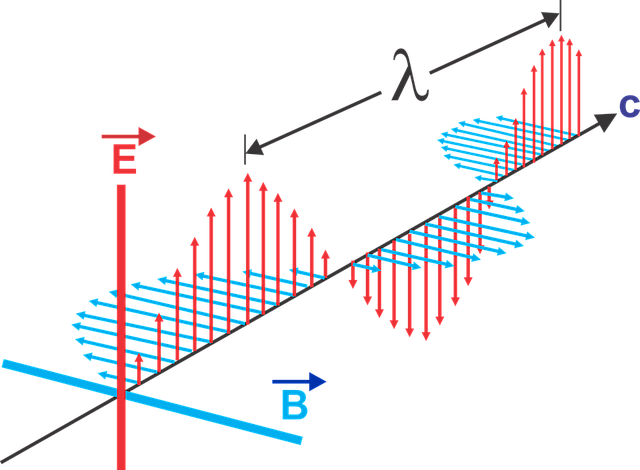
De Broglie hypothesis and matter waves :
De Broglie hypothesis state that the matter consist of both, particle nature and wave nature.
De Broglie wavelength (λ) is given as λ=h/p => λ=h/mv,
where,
'h' represents Plancks constant,
'p' represents particle,
'm' represents mass of the particle,
'v' represents velocity of the particle.
- From the above equation relation, it can be said that the wavelength of the matter is inversely proportional to the magnitude of the particles and linear momentum.
The De Broglie hypothesis is applicable to both Microscopic and Macroscopic particles.
The De Broglie is one of the equation that is used to define the wave properties of matter.
Electromagnetic radiation exhibit dual nature of both particle and wave.
