
I realise what I am doing here is probably old news to electrically orientated people but as a beginner my mind has been completely blown by how easy it is to generate sufficient voltage & amperage to power a light using inexpensive items most of us have in our homes already.
Five days ago I showed you how to do this using copper tube, screws & damp soil.

But ever keen to simplify and find for you the cheapest way to get things done I started playing yesterday with copper wire, tissue and tin foil.

Before this I had Esteban help me drill me some 1.5mm holes in a piece of wood, into which the copper wire will fit.

Then I rolled tissue over the copper wire and dunked it in water.

I covered the wet tissue with the tin foil and made sure the top end was bent in such a way that it would be able to connect with the next one.

With seven 'cells' I was able to get a reading of over 3V.
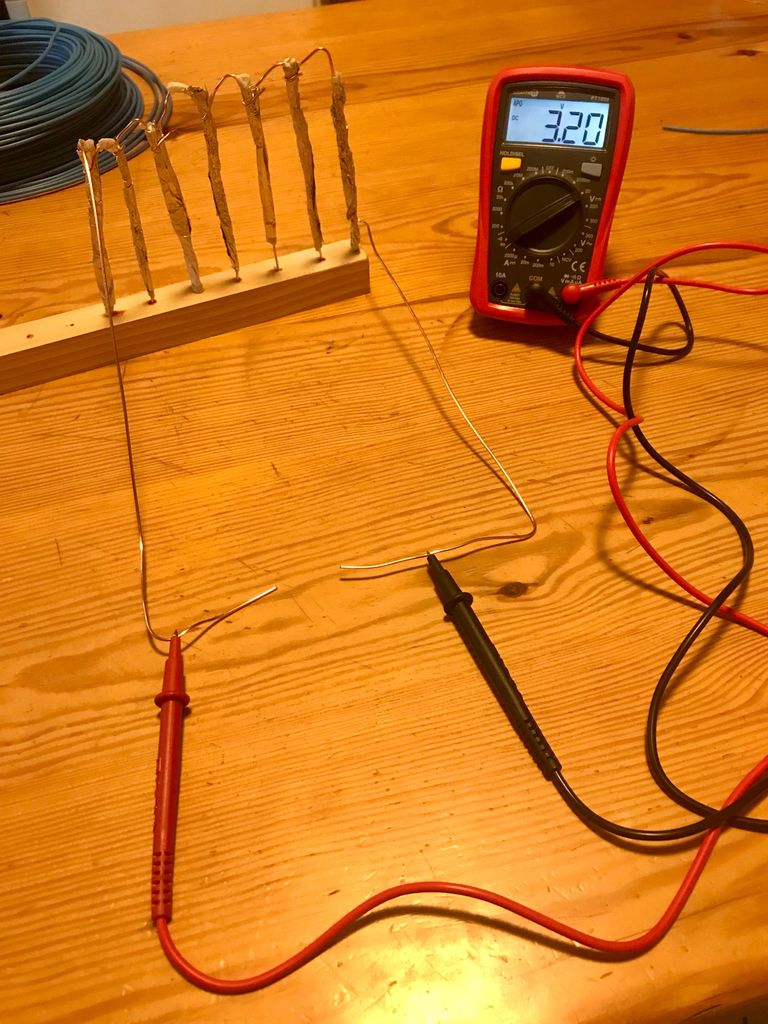
I understand now (thanks to the help of a few people) that to get a true representation of my device's output I should have the meter in series with the light I am intending to power but for the purposes of this demonstration I am keeping it simple.
Upon checking the amps I was disappointed to see that unlike my earth battery this was giving almost nothing at all. Just 0.01 of a milliamp. (Earth battery gave around 1mA).
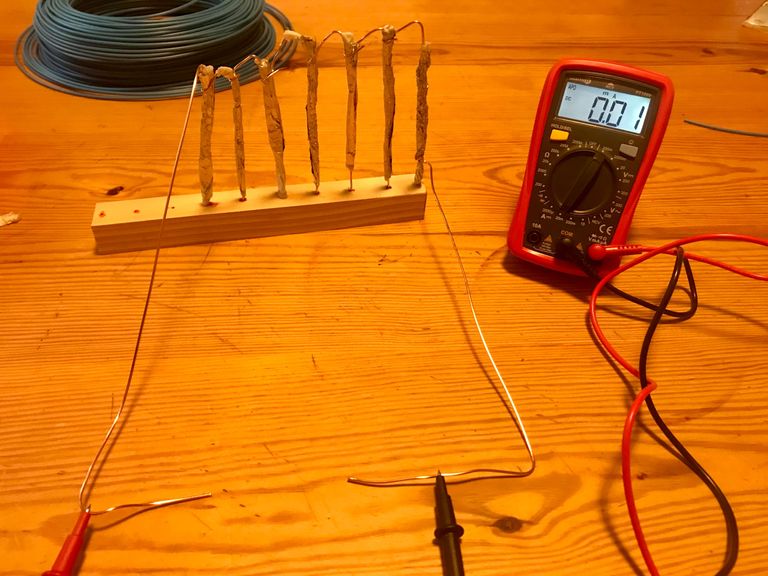
Even though this little light only needs 3V, it also needs a minimum of 1mA so with my current output I was unable to make it light up at all.

The missing ingredient
Have always been aware that salt water is more conductive than pure water but was trying to avoid using it because the main device I intend to build is ultimately destined for the garden where it will be buried in the soil. Salt water would have to be added regularly to maintain the higher output but this would have a detrimental effect on plants growing near the battery. And I don't want that. On the contrary, electricity generation in my soil should be having a very positive effect on the plants near by.
So again, for the purposes of this demonstration, to show you how easy this can be without any limitations, I added salt to the water and started from scratch with slightly longer sticks.
Instantly my amps jumped up to 1.29mA. A significant increase I think you will agree.
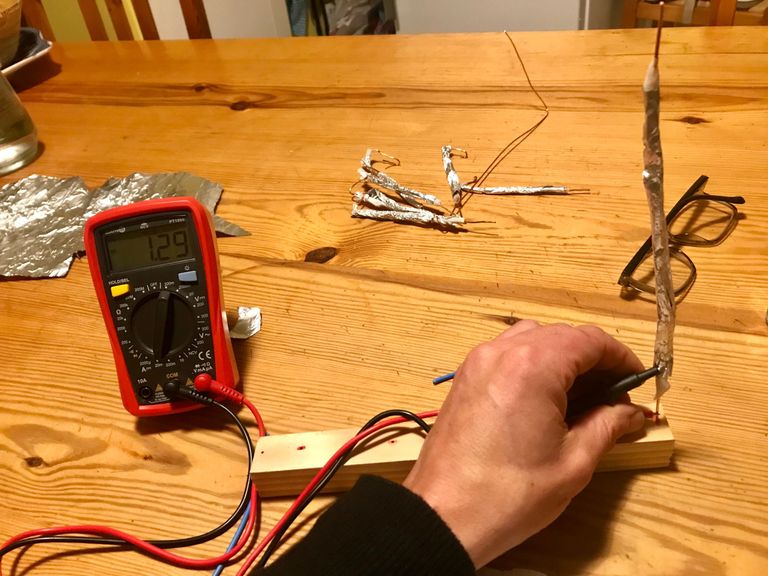
The voltage output didn't change with the addition of salt water and was still giving me 0.5V per cell so I set about making more, to get me up to the required 3V.

Just so you are clear, the amps won't increase while they remain wired in series like this. Only the voltage.
So with seven salt water cells I was able to power the light but due to the lack of amps it wasn't very bright.

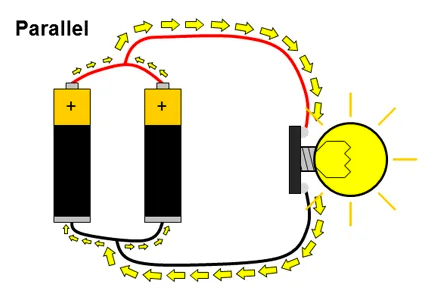
The solution to this problem is to wire the batteries in parallel, as shown here.
To get this done with a copper tube earth battery would have been much more work than with copper wire & tin foil so it took me very little time to create another row of seven cells in order to increase my amperage.
This worked exactly as expected, giving me double the amperage & brightness.

Here you can see where I have connected the two rows together on one side.

The tin foil is the anode and represents negative, always measured with the black end of your meter.
Viewed from the other side you can see where I have connected the two rows together at the top, copper to copper.

The copper is the cathode and represents positive, always measured with the red end of your meter.
Turing the lights off I was quite impressed by how bright it was and it occurred to me that you could even read a book by this light.

Which is just nuts when you think about it! How many people in poor countries have access to the relevant pieces to build this device but lack the knowledge to do so, having to rely on burning things to create light? Plenty I would imagine.
I also tested it out on this small LED.

Finding that it worked resoundingly well.

So the next time you think about buying solar in order to run a small light to illuminate your favourite reading book at night in an eco-friendly way, why not consider building one of these instead? It's way cheaper, doesn't take long to make, its only fuel is salty water and while I can't yet say for sure, something tells me it lasts a very long time, assuming you keep it 'charged'.

This little LED light normally takes one CR2032 lithium battery. They are not expensive as you can see here, but this doesn't at all make them an eco-friendly option.

The control group test
I intend now to install one of these lights as a permanent feature in the bedroom of my children and alongside it I will run another identical light, this one powered by the CR2032 battery. Over time these batteries will die and I will replace them. Over time my device will also die but like I said, this may not be for a long time, years or even decades? Hard to find info on this. (If you intend for your device to run for a long time it will be better to use 100% cotton in place of tissue).

I will however need to re-design it before my experiment begins to permit for an easier watering system. If it were sitting in a plastic tub of some kind this would be better. Then you could just periodically spray the tops and bottoms (where the tissue is exposed) with salty water without also spraying children's toys. For best functionality it could be enclosed entirely, preventing excessive evaporation.
In order for you to follow this experiment without me having to write multiple dedicated posts I will at the end of every post update you on two things:
- How many days the light connected to my device has been on.
- How many batteries the control group light has consumed (while also remaining on for the same period).
My experiment will conclude when my device becomes unable to turn the light on.
And we will see how many CR2032 batteries you can avoid buying by building one of these instead.
Can a device like this charge a phone?
I believe it can if you are keen and have some time on your hands, though it is not as simple as I imagined it would be in my last post on this subject because I did not have a full understanding of my multimeter. Since then I have been educated by a few electrically minded people and I understand now that mA (milliamp) is one 1000th of A (amp).
To charge a USB device like a phone we need 5V, so first we must extend our row of seven to around ten. Let's say it is ten for the sake of convenience.
We know that one cell gives us 1.29mA and a row of ten give us 5V. So with 2000 rows of 10 this will take my amps up to 2.5A (2580mA) which should I believe be enough to power most USB devices (once the step-up converted has been introduced) but this would mean making a total of 20,000 cells!
While I am genuinely keen to show you how this is possible, I am not that keen. I suppose if I pushed myself I could produce 1000 cells a day, meaning it would take 20 days to create a device which potentially charges my phone for free for years. Needless to say you could make the connections much closer together than I have done to save on space.
Out of curiosity I added salt water to one of my earth filled copper cells and the amps increase from 1mA to 10mA, fluctuation at times up to 20mA.

This means I would only need 2000 cells in total (potentially less depending on where that fluctuation settles) to achieve the required output for a USB device, but even this would be a big job. Expensive too.
Where do I go from here?
As I mentioned in a previous post, my goal is to create a device which powers a waterproof camera in the garden for three months, permitting me to prove the effectiveness of electroculture alongside a control group.
However, impractical is the word which comes to mind when imagining a device consisting of 20,000 cells. Though like I said, if you have the space and the inclination, it is unquestionably possible.
Personally I am keen to move forward in a more efficient manner and for this reason will now be looking at the capacitor sheet method, as described in this document.
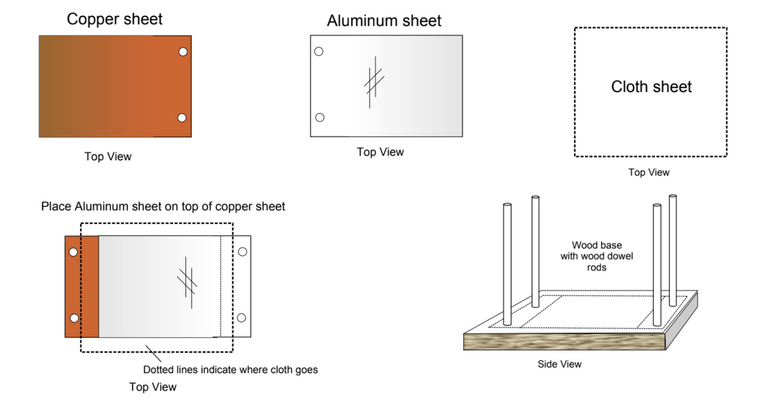
This is what they have to say about the capacitor sheet method:
This method is far better than using pipes or rods. By using copper and zinc or aluminium foil sheets you will get much more amperage out of your system! The amperage you will be collecting will be coming from 3 different sources. 1. The acid in the ground and water. 2. Energy that is being transmitted from the earth itself. 3. Energy that is being transmitted from the sky and space.
By the way, I want also to point out in this moment that it was this same document which encouraged me to make my first earth battery (the copper tubes) which they say will increase in voltage & amps significantly once they are buried in the earth. I've not done this yet because there is snow & ice on the ground currently and I don't fancy the idea of digging holes. But you can be sure I will put this claim to the test in the next few weeks and report on my findings here.

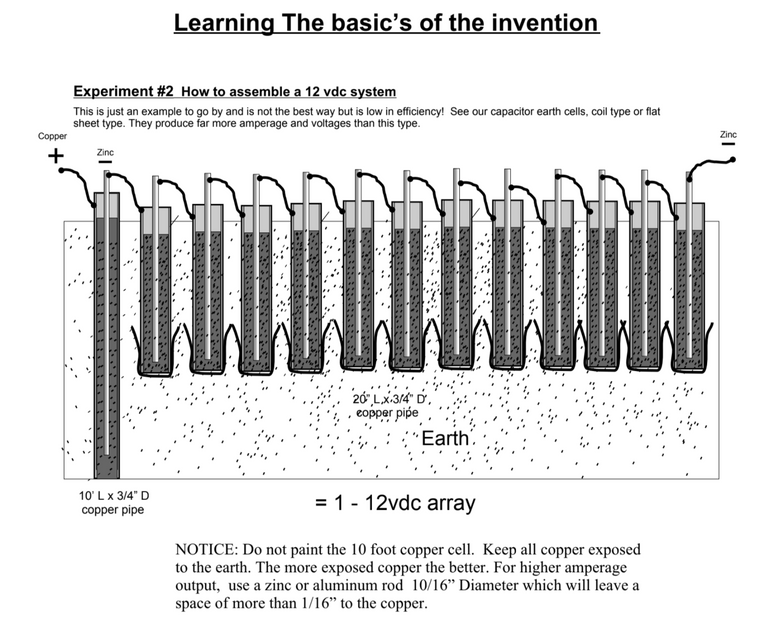
I have enjoyed working my way through this document which describes how one can place their copper tubes in the ground like this to produce a 12V system.
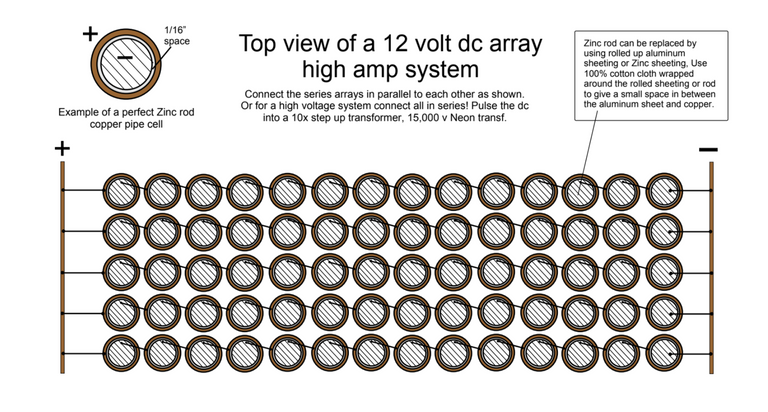
The third experiment can be seen here and offers a higher amperage.
In the interest of time I won't go into the others. Just the capacitor sheet method, which they say will endlessly increase in power the more sheets we add.
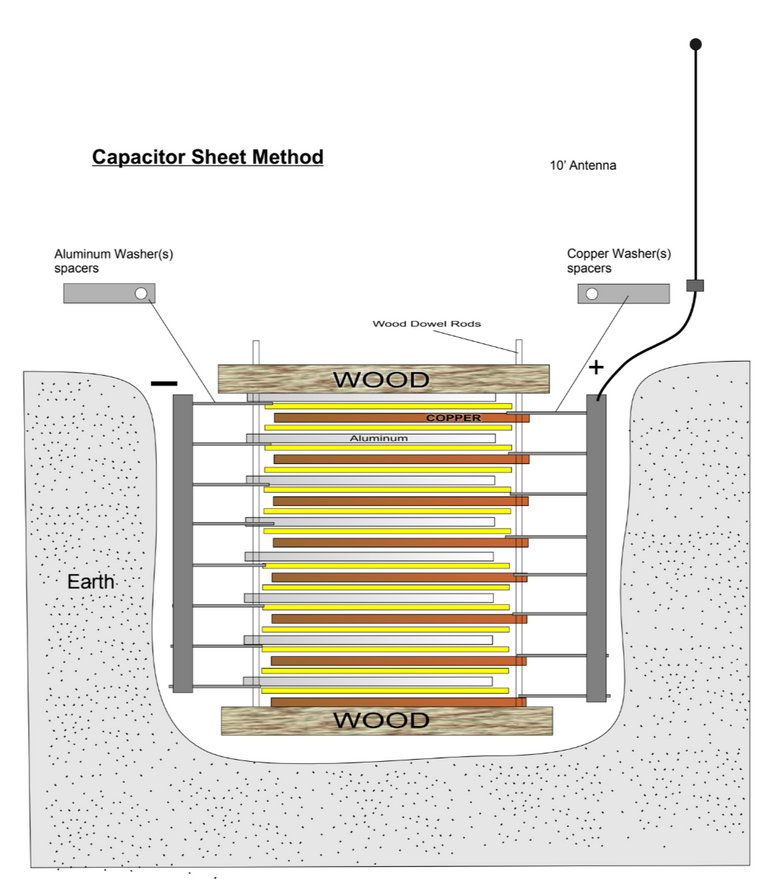
In this alternative design they have included a layer of dirt between the copper and aluminium sheets.
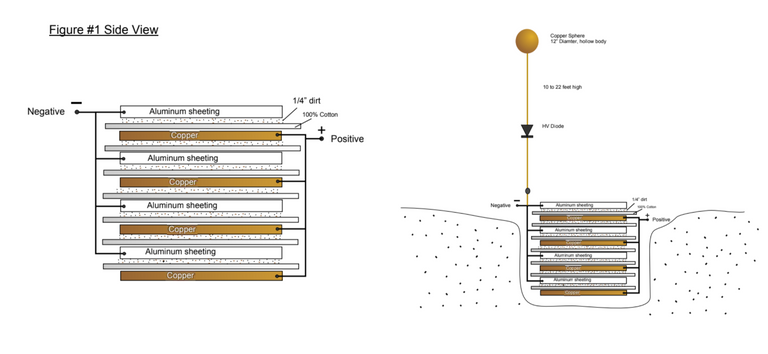
This all seems logical enough and I am excited now to get my hands on some copper sheets to build one of these and see what kind of readings I am getting on my meter.
As a capacitor this device can store energy while there is a current present (which theoretically should be continuous in this situation), so this makes it a very different kind of device to the copper tube earth battery or copper wire/tin foil battery which doesn't technically store energy. It just produces it for you constantly.
Final thoughts
I have concluded in recent days that all energy is in fact free. Viewed from another perspective one could also say that no energy is free. Indeed it is not possible to create a machine which collects energy without using any energy. So the only two questions which need to be asked are these:
1 - How much energy has been used to create my device?
2 - How much energy can my device produce during its lifetime?
If the answer to 1 is less than 2, the device is useful.
If you are intending to play with any of the ideas described in this post please tag me in to your findings when you post them. I really enjoy following the ripples of my influence here and perhaps, as my understanding of these subjects is constantly evolving, I may potentially be able to offer further suggestions which aid your progress moving forward.
Love & Light everyone 💡

Congratulations!
You have recieved a coconutty upvote! 🥥
Thank you for contributing to the Blurt Blockchain!
Keep up the great work!
Curated by @outofthematrix!
Please take a moment to vote for my witness! 🗳️ https://blurtwallet.com/~witnesses?highlight=outofthematrix