It is the most abundant element in the earth crust and also the fourth most abundant element in the universe. Even your DNA and RNA which is the code carrier of your life contains this very important element. Really 18.5% of your whole body is made of it.
Right now, an estimation of 80% of the world's energy are mainly compounds of this element. Ladies and gentlemen, allow me to qintroduce you to Mr Carbon!

Hahaha, don't mind me, I was trying to sound like one of those MC's, I hope I tried. Well, like I said, we will be discussing about the element carbon. When I learnt that this element seems to be at the center of everything that is going on in this universe, I felt the need to discuss this element with you guys. Practically everything about this element is interesting, I bet you will be thinking the same by the end of this article. Let just start with a bit introduction of this element, but before I do that let me explain some few words you might see too often in this article as you read along.
Isotope:- This is defined as the two or more forms of an element where the atoms have the same number of protons but different number of nuetrons.
Allotropy:- This is defined as the ability of an element to have distinctive different molecular structures in the same state. (i.e atoms of the element bonding in different ways at the same state).
Carbon has a symbol of C, If you have your periodic table with you, you can find it at group 14 and period 2. It is at the right hand side of the periodic table. I bet you have seen it. Alright let's continue.
It has an atomic number of 6 and a mass number of 12. Remember that I told us in one of our previous sections that atomic number is given by the number of protons in an atom, while the mass number is given by the sum of the protons and neutrons.
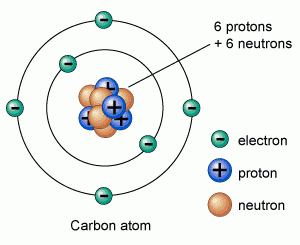
Using the KLMN representation, Carbon has two shells: the K and L shells, with the K-shell filled with two electrons and the other shell partly filled with only 4 electrons. Remember that K shell is filled with only 2 electrons while the rest of the shells, that is LMN are filled by 8 electrons. Due to this unfilled shell carbon can react with other elements. The display the an oxidation state that ranges from +4 to -4.
Carbons has only three naturally occurring isotopes, which are C-12 (which is most common and makes up 98.93% of the total carbon in the universe), C-13, and C-14 (which a radionuclide). Carbon also display allotropy in that at the same solid state it can exists either as an amorphous carbon, a graphite, or as a diamond. These allotropes behave differently.
A diamond is a crystal of tetrahedral shaped bonded atoms while a graphite has an hexagonal structure. Let's just consider and compare diamond and the graphite and see how they behaves totally differently (in their physical characteristics) due to their different molecular structures in the same state even though they are of the same element.
Diamond is the hardest known material in the world, while Graphite is one of the softest materials in the world.
Diamond is a very poor conductor of electricity and of the best insulators in the world, while Graphite is a good conductor of electricity.
Diamond is transparent while Graphite is opaque.
Diamonds are strong abrasives, while Graphite is a good lubricant.
Now that we have understood that their difference in molecular structure causes them to behave differently physically, let's us get to know if there is any interconnection between these allotropes.
We have already stated that the allotropes
of carbon are amorphous carbon, graphite and diamond. It is wise to classify these allotropes into crystalline and non crystalline.
Diamond and graphite are crystalline forms of carbon, while amorphous carbon is non crystalline.
Amorphous carbon is formed when materials that contain carbon do not have enough energy to burn completely. Being non crystalline, it has an irregular glassy state which are not held in crystalline microstructure. Amorphous carbon is the major constituent of charcoal, activated charcoal and soot (which can been seen in lamps). This black soot is used for making inks paints and rubber products. It can also used to form the core found in most dry cells.
For crystalline structure of carbon, at normal
temperature carbon takes the shape of graphite, which are good lubricant because of the arrangement of the atoms in its structure. Graphite are made commercially by treating petroleum coke in oxygen free oven.
When the pressure of graphite is increased to high point carbon takes a more compact allotropic form, which is the form of diamond. Diamond has a dentist that is higher than that of graphite. Each of the atoms in a diamond is bonded tetrahedrally to the 4 other carbon atoms surrounding it.

Diamond do have the same cubic structure as found in silcon and germanium and due to the strong carbon to carbon tetrahedral bond, diamond is the hardest known substance. Hardness means it can resist dent or scratch.
Most people think that because diamond is the hardest known substance in the world it is also the most stable substance. Some even say stuffs like "diamonds are forever". Well, that is a very wrong assertion, in fact diamonds are very unstable. Even at normal conditions they can transform into graphite. This shows that graphite are more stable than diamond.
Carbon is known to have the highest number of compound in the world because it doesn't find it hard to bond with other elements and even atoms of its self, and because of its valency it can form a multiple stable covalent bonds with multivalent atoms. Infact, a lot of the known chemical compounds are basically carbon compounds.
The sublimation point of carbon is the highest for any known element, thus it's really hard for carbon to sublime. Let me just explain what I meant by sublimation.
Sublimation is the process where by a substance changes directly from solid to gaseous state with out passing through the liquid phase.
Carbon has a melting point that is higher than that of metals like tungsten that are known for their high melting point, thus carbons remain solid at temperatures where these metals melt. Carbon is also known for its love for oxygen, It reacts with oxygen to form carbon oxides (like CO2 and CO), also it can easily remove oxygen from an element and reduce the compound to its metal. But despite its strong affinity for oxygen it's not easily oxidized, but rather carbon resist oxidation more than elements like copper, iron e.t.c.
It is worth noting that even though carbons are known to form a lot of compounds, most forms of carbon are unreactive. Normally at STP it doesn't react with tetraoxosulphateVI acid, hydrochloric acid, any alkali and chlorine gas.
Carbon has this special property of forming a very long chain of interconnecting carbon to carbon bonds. This property is called catenation and is not found in all elements. The carbon - carbon compounds formed by this catenation are very stable. It forms a lot of compounds through this way.
Carbon is the major constituent of the simplest form of an organic molecule, which is hydrocarbon. Hydrocarbons are mainly chains of carbons and a number of hydrogen bonded to these carbon atoms. The organic molecules' properties (i.e to say that the characteristics of the hydrocarbon) is affected by its functional group, side chains, chain length.
Compounds of carbon is found in basically every area of life, both living and non living thing. It is the study of carbon in organic life that is referred to as organic chemistry. When carbon combines with hydrogen and oxygen it forms important organic compounds like sugar, esters, alcohol and fats. Carbon is one of the constituent elements that forms the DNA and RNA which contains the total information about life.
In inorganic chemistry, carbon is also known for its ability to form carbides with reactive metals like tungsten.
Carbon is found in large amount in the sun, stars and comets. It is also seen in most planets and even traces of diamond have been seen in meteorite.
In our atmosphere carbon is not found in free state, rather it is seen in combination with oxygen in the form of carbondioxide. It is also found in water bodies in dissolved form. Carbon is also found in the biosphere.
Most sources of energy like coal, petroleum, natural gas contain carbon. The human body is made of carbon. If you around you, the woods you see, the plants and lots more contain carbon.
Even the carbondioxide we breath out (while plants breath in carbondioxide) is undoubtedly a carbon compound. Thus virtually everything in the universe is made of carbon.
Since we make use of carbon as energy and other stuff, it is wise to say that an increase in the number of automobiles and other human activities have lead to an unnecessary increase in the amount of carbon in the atmosphere. Thus, there must be a means by which nature balances all these to make the earth stable. This now takes us to carbon cycle.
Majorly, carbon cycle is just a cycle in which carbon is exchanged between the the atmosphere of the earth, biosphere, pedosphere, hydrosphere, geosphere
Since carbon makes up almost everything on earth, basically carbon cycle is just series of events that makes it possible for Earth to be able to sustain life. It also describes how carbon is reused throughout the biosphere.
Below is a diagram explaining how carbon is recycled.

Almost everything is made of carbon, thus carbon has so many applications. I will be listing only a few in this discussion.
Graphite which is carbon, in conjunction with clay is used in making of pencil leads. Graphite also serves as lubricant.
Cellulose (which contains carbon) is used primarily for maintaining the structure of a plant.
Diamonds are used in jewelry, also industrial diamonds are used for cutting, drilling, grinding, polishing of metals and stones.
Carbon black is used for producing printing ink, carbon paper e.t.c
Activated charcoal which is a well know absorbent is used in filter material. it is used in water purification and making of gas mask
Plastics in general are made of synthetic polymers of carbon.
Carbon is used as electrodes in electroforming and electroplating.
Woods, oil and coal are sources of fuel and they produce heat and light energy when they are used.

- Carbon (as coke) is used to reduce metals like iron to their ore.
As you can guess, carbon in its elemental form is not toxic since everything in the world is not toxic, but carbon in form of carbon black can be hazardous to health. People that are engaged in works that involve the production of carbon black are found to suffer from pneumoconiosis which is a diseases of the lungs caused by inhalation of particles.
This might lead to permanent or temporary damage of the lungs. Also exposure to carbon soot causes skin conditions which includes oral mucosal lesions and inflammation of the hair follicles.
People most times assume that carbon is only found in energy sources like wood charcoal, crude oil e.t.c, not knowing that carbon is found in the smallest of things, even in our DNA and RNA. Because of that, they fail to realize the beauty and the great importance of this element.
The major idea of this discussion is to broaden our knowledge about this element, since basically everything around us is made of this element. Right now we know that carbon is contained in cellulose, and all forms of sugar (glucose, sucrose e.t.c) are compounds of carbon.
In this article we listed some applications of carbon, but it doesn't end there, there are still so many left and more applications are yet to be discovered. It is alleged that carbon is the most researched element in the universe. Well, I don't really know about that, but one thing I am sure about is this; there is nothing in this world that should be more researched on than the element that is at the center of everything, and is the basic building block of life.
REFERENCES
Great work @whileponderin.
Thanks @epistem