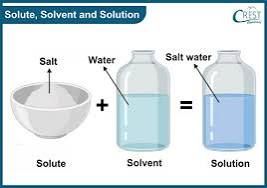
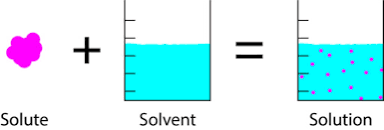
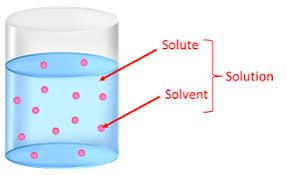
A solution is a mixture of substances.
A Solute is a dissolving substance. For example a lump of sugar which dissolves and disappears in a glass of water.
Other examples of solute includes, table salt, blue copper sulphate, Alum salt dissolving in water, Barium chloride salt and so on.
The important aspect to be noted as regards solute is that they get dissolved in another substance which is known as the SOLVENT.
On the other hand, a solvent is a substance that dissolves another substance or in which other substances get dissolved.
A typical example of a solvent is water. Water is otherwise referred to as a universal solvent. Water as a solvent can dissolve almost every solute in the universe. Other examples of solvent includes; ether, organic solvents that can dissolve plastic materials. Also kerosine can also dissolve some organic materials and so on.
Last but not least, Solution is a mixture of materials which include the substance that dissolves in another substance to give rise to the mixture in question.
A solution comprises a mixture of solute and solvent such as a lump of sugar that dissolves in water thus forming a sugar solution.
A sugar solution contains a sugar that has been dissolved inside water.
Other examples of solutions include; salt solution, Calcium hydroxide solution, Silver nitrate solution, Sodium chloride solution, sulphuric acid solution, alkali solution and so on.
The important thing to note is that a solution is a mixture of constituents including solute and solvent.
A solution is usually transparent, which means it can be viewed through. Transparency means another area can be viewed directly through a solution. This transparency has nothing to do with colour as a sugar solution is colourless does not mean that other colourful solution will not be transparent.
A blue copper sulphate solution is also transparent because it also permits viewing through a glass test-tube.
Conversely, a milk is not regarded as solution because it is opaque. As everyone knows, an opaque object does not permit a clear view or allow viewing due to inability of light to pass through talkless of being reflected.
Therefore a milk is not a solution and it is more of an emulsion.
Last but not least as regards this post on solution, solute and solubility. Some people tends to confuse solution and melting together, however the two are different as for a solution to be formed it must involved a Solute dissolving in a solvent giving rise to a transparent liquid but in the case of melting it only involves a single substance without having to dissolve in another substance.
Melting only has to do with a change in temperature of a substance for example a melting ice cream once it is heated or kept in an environment with high temperature, or heated sugar that melts to form a dark liquid substance or heated salt that is placed on a Bunsen burner and so on.
That's all for now as regards solution, solute and solvent and happy blogging
Happy Blogging and Reading 💥💥💥💥
Video from learning junction YouTuber